Force Field Functions
Bonded Functions
Bonded terms are defined by atoms that are directly bonded - bond lengths, bond angles, dihedral angles, improper dihedral angles and out-of-plane angles. These terms are also referred as "Valence terms".
Bond Functions
Bond is defined by two atoms:

| ID | Function | Parameters |
|---|---|---|
| BHARM | ||
| BQUAR | ||
| BDQUA |
Bond Angle Functions
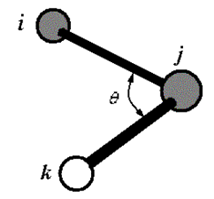
Bond angle is defined as by three atoms i-j-k:
| ID | Function | Parameters |
|---|---|---|
| AHARM | ||
| AQUAR | ||
| ADQUA* | ||
| ACOSH |
*
Dihedral Angle Function
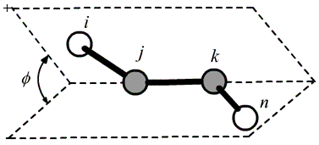
dihedral angle is defined by atom i-j-k-l, where j-k is the center bond.
| ID | Function | Parameters |
|---|---|---|
| TBCOS* | ||
| TCOSM | ||
| TCOSP | ||
| TCS3M |
*
Improper Dihedral Angle Function
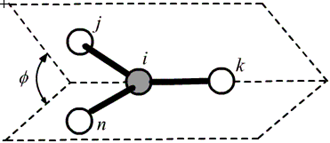
The improper dihedral angle is measured as the dihedral angle of two surfaces made by bond pairs of j-i/i-k and n-i/i-k respectively.
| ID | Function | Parameters |
|---|---|---|
| IHARM | ||
| IBCOS |
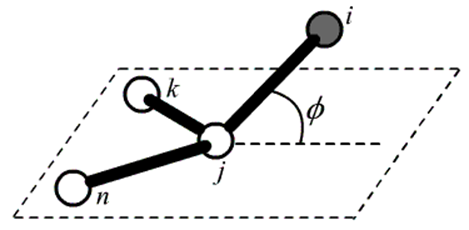
Out-of-Plane Angle
| ID | Function | Parameters |
|---|---|---|
| OHARM | ||
| OBCOS |
Cross-coupling Functions
The cross coupling terms are used in CFF and TEAM force field types. These functions describe the coupling of two or three bonded terms.
The reference values of bond (
| ID | Description | Function | Par |
|---|---|---|---|
| C_B_B | Two bonds with a common atom | ||
| C_B_A | A bond angle with one of the two bonds forming the angle | ||
| C_A_A | Two angles share a common bond | ||
| C_B_T | A dihedral angle with its center bond | ||
| C_A_T | A dihedral angle with one of its two angles | ||
| C_BBT | A dihedral angle with of one of its two side bonds | ||
| C_AAT | A dihedral angle with of its two side angles | ||
| C_B2B | Two side bonds in a dihedral angle |
Nonbonded Functions
Electrostatic Function
The electrostatic interactions are interactions between atom i and j that are either in different molecules, or in one molecule but separated by at least three consecutive bonds (1 - 4 and beyond). The energy is given by Columbic function:
charges
by using two parameters:
| ID | Description | Par |
|---|---|---|
| ATC | Atom type charge | |
| BINC | Bond increment charge |
Lennard Jones Function
LJ-12-6 is commonly used.
The combination rule is
Therefore, the energy is calculated using two parameters for each atom
| ID | Description | Par |
|---|---|---|
| N12-6 | LJ-12-6 parameters |
LJ-9-6 is used by CFF:
where the combination rule is
Therefore, the energy is calculated using two parameters for each atom
| ID | Description | Par |
|---|---|---|
| N_9_6 | LJ-9-6 parameters |
Other Function
General LJ-N-M: (n>m)
The combination rule is
Buckingham
Morse function (BMORS):
Functions and Parameters
The above function in different force field types are shown as examples in the following table.
| FFType | Function ID | Atom types | Parameters |
|---|---|---|---|
| All | ATC | o_1-/2 | -0.5 |
| BINC | c_4, o_1 | 0.2360 | |
| TEAM | BDQUA | c_2, c_2 | |
| ADQUA | c_2, c_2, c_4 | 179.9511, 53.8431 | |
| TBCOS | c_3, c_3, c_4, h_1 | 1, -0.1978, 2*, 1.0728, 3*, -0.0947 | |
| TBCOS | c_4, c_3, c_3, c_4 | 2*, 5.4661 | |
| OHARM | c_3a, c_3o, c_4, o_1 | 0.0000, 44.0651 | |
| N12_6 | c_3 | 3.9800, 0.0760 | |
| C_B_B | c_2, c_2, c_4 | 8.6266 | |
| C_B_A | c_3, c_4, o_2 | 51.9669 | |
| CFF | BQUAR | c_2, c_2 | 1.2107, 1212.6459, -2425.2919, 2829.5072 |
| AQUAR | c_2, c_2, c_4 | 179.8087, 53.8431, -10741.8351, -2569.8855 | |
| TCS3M | c_2, c_2, c_4, c_4 | 0.1000, -0.2500*, 0.1200 | |
| OHARM | c_3, c_3, c_4, c_4 | 0.0000*, 2.3488* | |
| N_9_6 | c_2 | 3.9015*, 0.0819* | |
| C_B_B | c_2, c_2, c_4 | -4.6381 | |
| C_B_A | c_34, c_44, h_1 | 21.4453* | |
| AMBER | BHARM | c_2, c_2 | 1.2057*, 1276.7505* |
| AHARM | c_2, c_2, c_4 | 179.9975*, 21.7689* | |
| TCOSP | c_3, c_3, c_3a, c_3a | 180.0*, 0.9349*, 2*, 0.0*, 0.0968*, 3* | |
| IBCOS | c_3, c_4o, c_3, h_1 | 180.0000*, 0.0000 , 2.0000* | |
| N12_6 | CC2H2 | 3.9300*, 0.0660* | |
| CHARMM | BHARM | c_2, c_2 | 1.2057*, 1276.7505* |
| AHARM | c_2, c_2, c_4 | 179.9975*, 21.7689* | |
| TCOSP | c_3, c_3, c_3a, c_3a | 180.0*, 0.9349*, 2*, 0.0*, 0.0968*, 3* | |
| IHARM | c_34, c_34, c_44, h_1 | 0.0000*, 73.6481* | |
| N12_6 | n_3 | 3.6930*, 0.1450* | |
| V14LJ | c_2 | 3.8250*, 0.0910* | |
| DREIDING | N12_6 | c_4 | 3.9300*, 0.0660* |
| BHARM | c_4, c_4 | 1.5283 , 213.6099 | |
| ACOSH | c_4, c_4, h_1 | 107.1210 , 54.6774 | |
| TCOSM | h_1, c_4, c_4, h_1 | 0.0000*, -0.1405 , 3.0000* | |
| OCOSH | c_3, n_3, c_4, h_1p | 0.0000*, 10.0000* |